- HOME
- Pipeline
(As of October 1, 2025)
| Cell type / Code | Application | JP/US | Pre-clinical | Clinical Research | Phase 1/2 | Phase 3 | NDA | Approval |
|---|---|---|---|---|---|---|---|---|
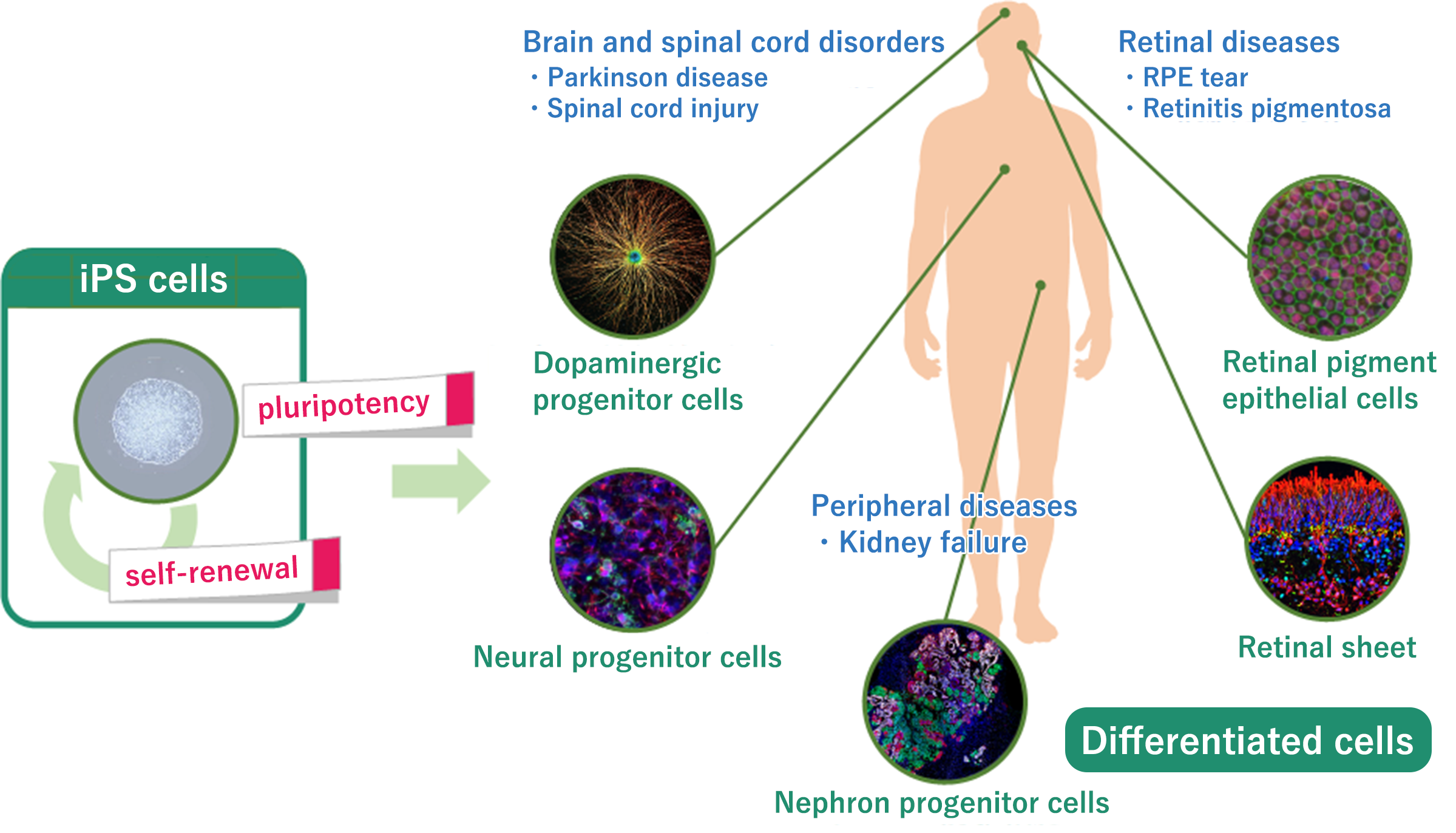
| Dopaminergic progenitor cells (Allo) CT1-DAP001/DSP-1083 |
Parkinson’s disease |
JP US |
4 5 |
1 |
||||
| Retinal pigment epithelial cells (Allo) HLCR011 |
Retinal pigment epithelial tear | JP | 5 | |||||
| Retinal sheet(tissue) (Allo) DSP-3077 |
Retinitis pigmentosa |
JP US |
2 |
5 |
||||
| Neural progenitor cells (Allo) SMP-0115 |
Spinal cord injury |
JP US |
3 |
|||||
| Nephron progenitor cells (organ) (Auto/Allo) |
Kidney failure | JP/US |
1:Kyoto University Hospital
2:Kobe Eye Center 3:Keio University Hospital
4:University of California, San Diego 5:Sponser initiated clinical trial